Research
Innovations in vaccine research have been influenced by the emerging field of ‘immunoengineering,’ which describes collaborative efforts between engineers and immunologists to study and modulate the immune system. We are particularly invested in the development of supramolecular peptide nanofibers as self-adjuvanting and inflammation-free vaccine carriers. These fibrillar scaffolds are attractive due to the ability of peptides to fold into specific secondary structures such as a-helices and ß-sheets and the rich chemistry with which their function can be manipulated. The highly modular nature of supramolecular assembly allows one to mix multiple functional moieties (antigens, adjuvants, cytokines, etc.) with stoichiometric precision and offers a powerful platform for the development of therapies with fine-tuned immunogenicity.
Effects of chirality from molecules to materials
Chirality is a simple geometric property that describes molecules that are not superimposable with their mirror image and one of life’s most distinctive signatures is its high selectivity for chiral molecules, like L-amino acids and D-sugars. Many biochemical processes are driven by the chirality of molecules, which play a crucial role in maintaining normal functions in organisms. In recent years, there is an emerging interest in the introduction of chiral effects into nanomaterials and one of the most rapidly expanding areas is the manipulation of molecular chirality to control biological outcomes. We are interested in understanding how chirality impacts the self-assembly of peptides, molecular packing, and immunological outcomes.
Mechanisms of adjuvant activity, toxicity, and clearance of supramolecular peptide nanofibers
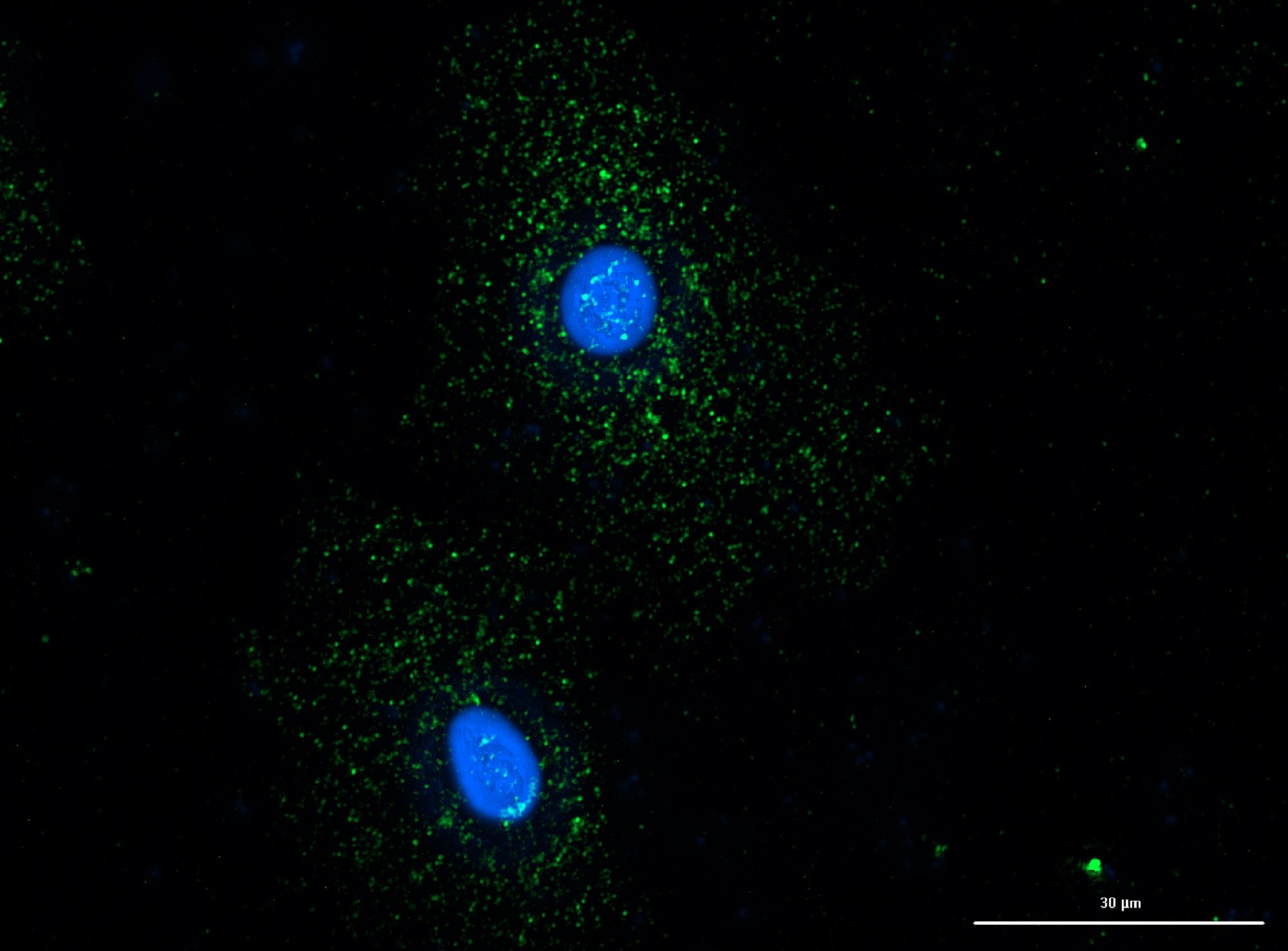
Once implanted in vivo, nanomaterials begin to interact with the phagocytic cells of the immune system such as macrophages (MΦs) and dendritic cells (DCs). The immune system has evolved to protect us from foreign materials, and it may or may not be desirable for nanomaterials to engage with the immune system depending on the application. In particular, peptide-based nanomaterials are problematic as they are prone to immune recognition. If the objective of the nanomaterial is to regenerate tissue or target therapies, then unscheduled clearance by the immune system should be avoided. On the other hand, if the immune system is the target then nanomaterials must be designed to elicit the appropriate level and phenotype of immune responses.
Numerous studies using different self-assembling peptide sequences have shown that peptide nanofibers are recognized and internalized by immune cells and trigger mechanisms of innate immunity making them promising nanomaterials for cell-free vaccine development. Unraveling the nature of these interactions on how nanomaterial chirality impacts innate immunity is crucial for informing future research applications and balancing immunogenicity with safety. Chirality is subtle in that chiral analogs do not change side-chain size, flexibility, hydropathy, charge, or polarity and allow the unbiased study of cell nanomaterial interactions.
Peptide nanofiber-based boosters to augment protection induced by clinically approved vaccines
A century since the first use of BCG, the first and only approved vaccine to prevent Mycobacterium tuberculosis (Mtb) infection, and over a billion administrations of the vaccine, Mtb remains the number one killer by infectious disease in the world. In 2019, there were approximately 10 million new cases of tuberculosis infection, and 1.4 million people died of Mtb disease (WHO, 2020). As a result of the high burden of TB, the development and spread of drug-resistant strains of M. tuberculosis remain an imminent threat to global health.
Numerous studies have shown that T lymphocytes are a critical component of a protective immune response against Mtb infection. More specifically, a subset of memory T cells identified as tissue-resident memory T cells (TRM) were demonstrated to have protective effects upon adoptive transfer into Mtb-infected animals. These cells are characterized by surface markers, most notably CD44hi CD62Llo CD69+ CCR7-, as well as tissue-homing markers, and are shielded from i.v. anti-CD45 staining. Importantly, TRMs have been correlated with protection in a study demonstrating the protective efficacy of intravenous BCG in NHP models of tuberculosis infection.
Our previous work has shown that the KFE8 nanofibers promote antigen presentation through autophagic mechanisms (Rudra, 2017) and can induce antigen-specific T cell and B cell responses, as well as polyclonal effector T cells (Chesson, 2018). Based on these previous studies and the recent advances that identify a protective role for Mtb-specific TRM T cells, we are interested in the development of peptide nanofiber-based pulmonary vaccines that would enhance BCG-primed immunity in a heterologous prime-boost strategy by promoting the generation of TRM pools in the lung parenchyma.
Molecular mechanisms of peptide nanofibers/TLR agonist combination adjuvants
Preliminary studies on mechanisms of action indicate that, unlike pathogen-associated molecular patterns (PAMPs) (toll-like receptor (TLR) agonists), peptide nanofibers do not cause DC maturation but up-regulate scavenger receptors and facilitate the release of damage-associated molecular patterns (DAMPs) related to osmotic/oxidative stress. The key advantage of peptide nanofibers over emulsion adjuvants like alum or QS21 is that the primary sequence of the self-associating peptide can be designed to control the physicochemical features such as morphology, charge, chirality, or hydrophobicity, which are key contributors to adjuvant activity.
Due to their profound immunostimulatory effect on DCs, researchers have combined TLR agonists with peptide nanofibers to improve the functional spectrum of vaccine-elicited responses. However, the mechanisms of innate immune activation by these combinations have not been elucidated. A strong rationale for combining peptide nanofibers with PAMPs is based on their individual mechanisms of action and regulatory approval of DAMP-PAMP combination adjuvants (e.g. AS04). We are interested in understanding how molecular mechanisms of DAMP-inducing peptide nanofibers and TLR agonist combinations orchestrate innate immune signaling and responses that are complementary, synergistic, or inhibitory for balancing immunogenicity with safety.